The CoolMini Applicator for the Submental Region
The new CoolMini applicator is uniquely designed for small areas of fat, including the submental area. Over 500 CoolMini treatments have been performed to date as a result of pilot clinical work, clinical trials and our controlled European launch that commenced in June. The results, achieved in as little as 1-2 office visits, have been very encouraging with very high patient satisfaction and the same level of efficacy achieved with other CoolSculpting applicators.
Here are a few facts from my notes taken during training a couple of weeks ago in relation to this treatment area:
- 7.2 Million people would consider a non-surgical alternative for chin fat
- 2014 ASDS survey, 68% of respondents said they wanted to reduce fat under their chins
- 3rd highest area of patient concern
Submental Before & After Results
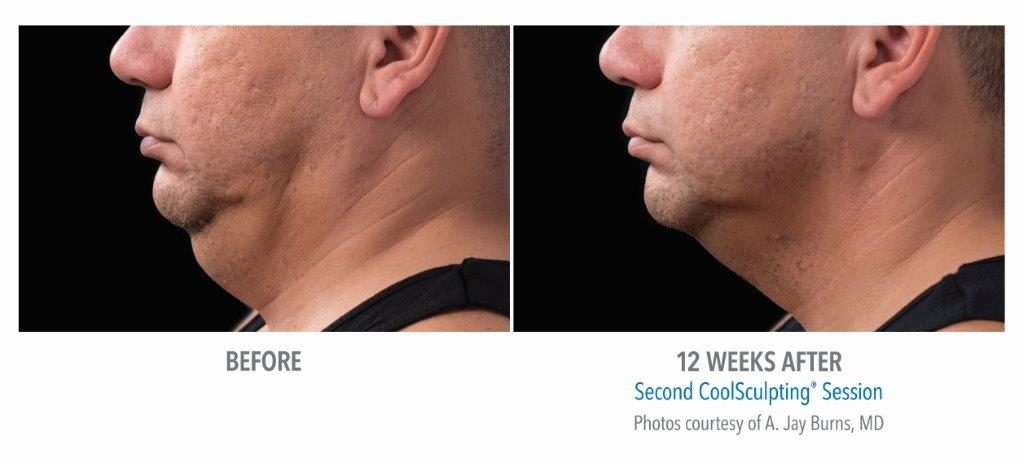
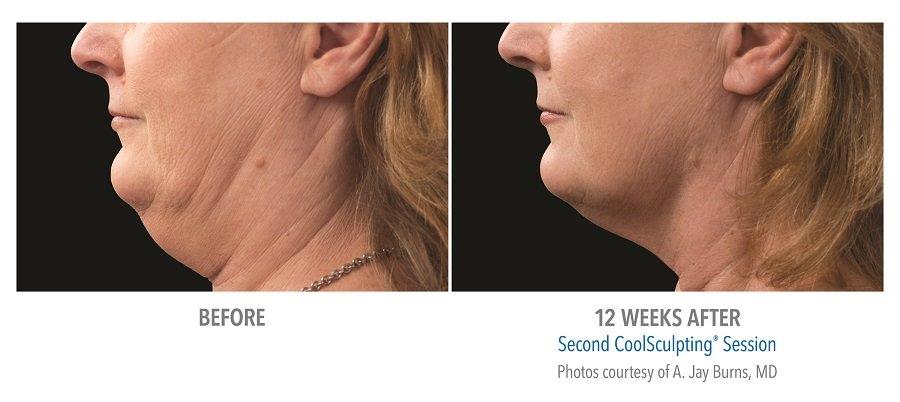
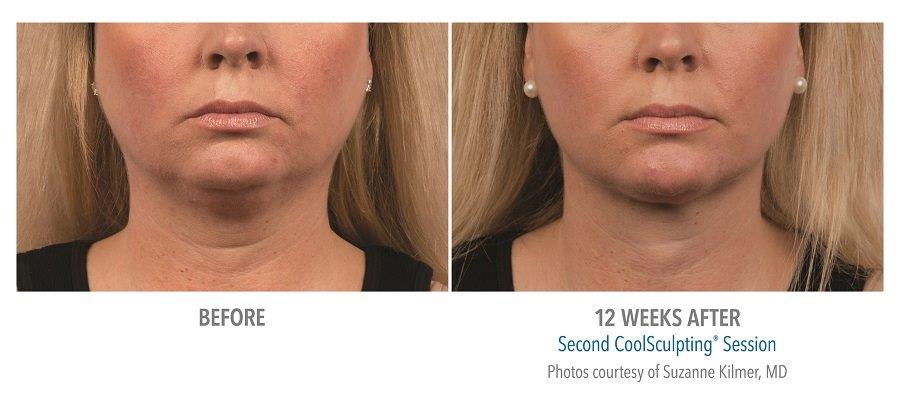
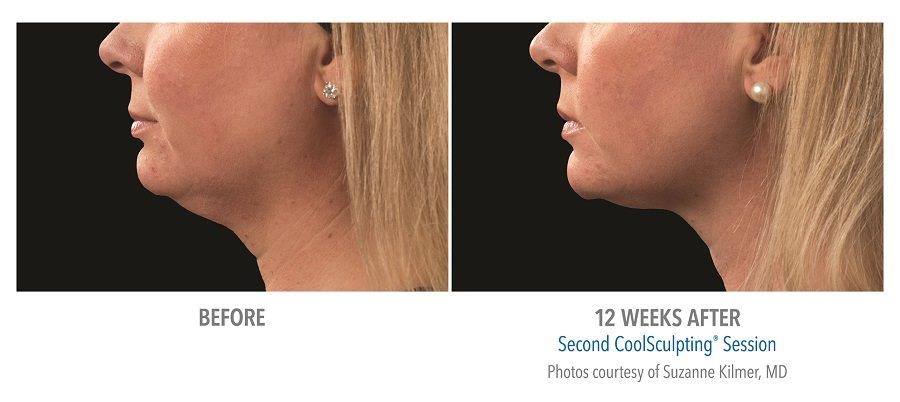
A 2014 study conducted by The American Society for Dermatologic Surgery (ASDS) showed that approximately 67% of people were concerned with chin and neck fat, consistent with the previous year. Additionally, a recent study conducted in the United States revealed that 22.4 million people are interested in seeking non-invasive fat reduction solutions.
“Many of my patients are frustrated with the fat they develop in the chin area because it is often hard to eliminate based on lifestyle changes alone, and the only available options to treat this area have been very invasive,” said Roy G. Geronemus, M.D., Director of the Laser & Skin Surgery Center of New York. “My practice has used the CoolSculpting procedure for many years to successfully reduce unwanted fat, and we are glad that we can now leverage this same technology to comfortably and non-surgically treat the double chin.” Read full press release
Would you like to treat extra fat in those hard to remove spots? Call us to schedule an appointment today!Vienna, Virginia Tel: (703) 982-74307611 | Richmond, Virginia Tel: (703) 982-7430
Results and patient experience may vary. In the U.S., the CoolSculpting procedure is FDA-cleared for the treatment of visible fat bulges in the submental area, thigh, abdomen and flank. The CoolSculpting procedure is available worldwide. ZELTIQ, CoolSculpting, the CoolSculpting logo, and the Snowflake design are registered trademarks. CoolMini is a trademark of ZELTIQ Aesthetics, Inc. © 2015. All rights reserved. IC2064-A